Abstract
Background
VOD/SOS is an unpredictable, potentially life-threatening complication of conditioning regimens for HSCT or of chemotherapy without HSCT. VOD/SOS with multi-organ dysfunction (MOD; typically defined as renal and/or pulmonary dysfunction) may be associated with survival of 20%-30% (Coppell et al. Biol Blood Marrow Transplant. 2010;16[2]:157-168. Richardson et al. Blood. 2016;127[13]:1656-1665. Strouse et al. Biol Blood Marrow Transplant . 2016;22[7]:1306-1312). For adult and pediatric patients, defibrotide (25 mg/kg/day in 4 divided doses) is approved to treat hepatic VOD/SOS with renal or pulmonary dysfunction post-HSCT in the United States, and to treat severe hepatic VOD/SOS post-HSCT in patients over 1 month of age in the European Union. The overall efficacy and safety of defibrotide treatment in adult and pediatric patients with VOD/SOS have been reported in controlled and uncontrolled studies, including a large expanded-access study and a historically controlled phase 3 trial. Reported here is a pooled Day +100 survival analysis of available data from defibrotide studies in patients with VOD/SOS with or without MOD.
Methods
A title or abstract search of PubMed, Embase, and relevant 2017 congresses was performed for "defibrotide" in the treatment of VOD/SOS. Prospective and retrospective studies, post-HSCT and post-chemotherapy without HSCT, were included in the analysis, but case reports of fewer than 10 patients were excluded. Citations were reviewed for relevance to this analysis, and the resulting studies were divided into subgroups to analyze data for patients with and without MOD. A random effects model was used for pooling data for efficacy. Interstudy heterogeneity was assessed with Cochran's Q-test. The percentage of total variation across studies due to heterogeneity was evaluated by the I2 measure. Safety was also evaluated.
Results
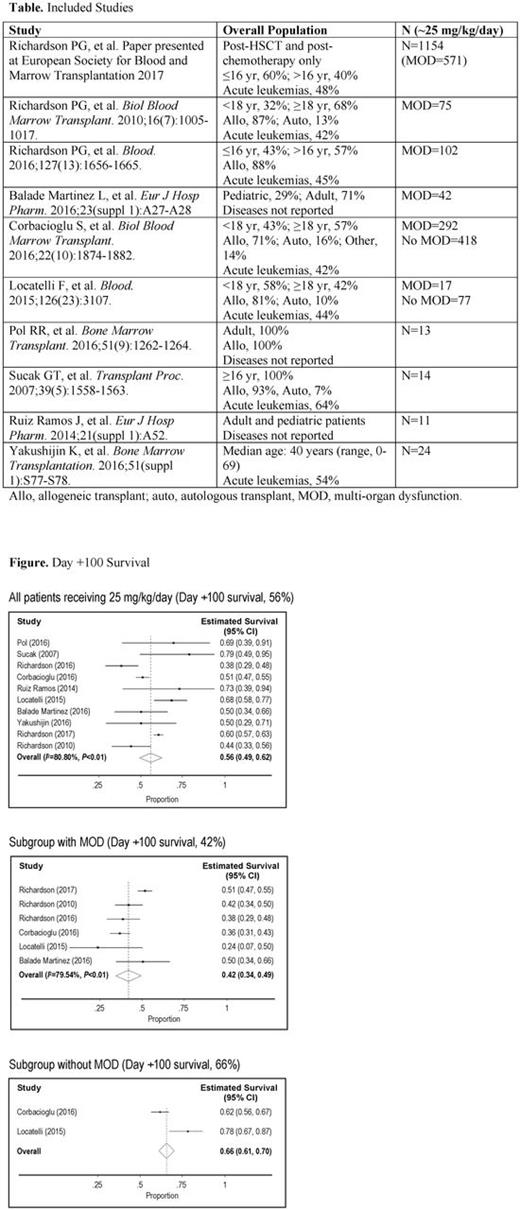
The literature review identified 606 publications and abstracts; 581 were excluded for relevance. This yielded 25 records (16 manuscripts, 9 abstracts), which were further refined by exclusion of 8 reports that were post hoc analyses, reviews, duplicates, case reports, or a prevention trial. Of the remaining 17, 10 records included data for 2239 patients, nearly all of whom were treated with ~25 mg/kg/day, including 6 that specified patients with MOD (1177 patients), 2 of which also specified patients without MOD (495 patients), and 4 where MOD status was not reported (Table). The other 7/17 studies included off-label dosages or the dosage was not reported. Estimated Day +100 survival for patients receiving defibrotide ~25 mg/kg/day across all studies was 56% (95% confidence interval [CI], 49%-62%; Figure). Pooled subgroup results showed estimated Day +100 survival rates of 42% (95% CI, 34%-49%) in the MOD subgroup; and 66% (95% CI, 61%-70%) in the no MOD subgroup (Figure).
Safety results were not pooled for these studies due to differences in safety reporting methodology, but results of individual studies were generally consistent with the safety profile found in the phase 3 historically controlled trial in VOD/SOS patients with MOD (Richardson et al. Blood. 2016;127[13]:1656-1665). The trial reported that all but 1 of the 102 defibrotide treated patients and all 32 HC patients experienced ≥1 AE. Hypotension was the most frequent AE (39% for defibrotide, 50% for controls), and common hemorrhagic AEs, which included pulmonary alveolar and gastrointestinal hemorrhage, occurred in 64% of defibrotide-treated patients and 75% of controls. Related AEs in the defibrotide arm included hemorrhagic events and hypotension.
Conclusion
In this pooled analysis of studies of defibrotide given primarily at the approved dose of 25 mg/kg/day for the treatment of VOD/SOS, estimated Day +100 survival was 56% in the 2239 patients with or without MOD. As expected, survival in patients with VOD/SOS without MOD was greater at Day +100 (66%) than in patients with VOD/SOS with MOD (42%). Safety results in the individual studies were generally consistent with the known safety profile of defibrotide.
Support: Jazz Pharmaceuticals.
Richardson: Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides AB: Membership on an entity's Board of Directors or advisory committees. Aggarwal: Novel Health Strategies: Employment, Other: Funding from Jazz Pharmaceuticals. Topaloglu: Novel Health Strategies: Employment, Other: Funding from Jazz Pharmaceuticals. Villa: Jazz Pharmaceuticals: Employment, Other: stock options. Corbacioglu: Jazz Pharmaceuticals: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal